Introduction
Manufacture
of particles is an important activity in many industries such as pharmaceutical,
cosmetics, food, printing and analytical sciences. Essentially, particle
manufacturing technology is required to produce particles in a defined size
range, to enhance solubility, to provide protection to a core, to alter the
characteristics of a substance in a particle core, to alter the interaction of a
core with its surroundings, to target a particle to a specific site or to ensure
that the core substance is only available under defined conditions.
Pharmaceutically the need is often to enhance stability, solubility or release
characteristics of an active substance, or to enable targeting of an active
substance or provide convenience in administration. Many existing procedures are
lengthy, of poor efficiency or have poor reproducibility. RCSI has developed a
versatile and efficient process that addresses the production of complex
particles in a single-step with efficient incorporation of the active
components.
Controlled-release technologies remain an important tool for
pharmaceutical companies for enhancing the efficacy, improving patient
compliance and to protect their branded products from generic market. The
controlled-release market was worth nearly US$21 billion globally in
2008 and is expected to reach US$27 billion in 2017. There is also a clear opportunity for new enhanced
formulations of biological therapeutics which will extend their half life, offer greater patient compliance and
reduce associated healthcare cost. The use of nanoparticle technologies for poorly soluble drugs for
enhancing their solubility represents a major and important
opportunity for the pharmaceutical industry with potential benefits of improved
efficacy, safety and better patient compliance. Approximately 50% of marketed drugs and as many drugs in the pharmaceutical developmental pipeline present solubility issues.
The nanotechnology drug delivery market is expected to grow for the
period 2009-14, to reach almost $16bn by 2014.
Technology
The
School of Pharmacy at RCSI has developed a novel microparticle engineering (MPE)
technology. The
process is based on spray drying, using specifically designed concentric nozzle
system so that particles emerge ready-formed in a process readily adaptable to
manufacturing environments (Figure
1). Particle ranging in size from <1 micron to >100microns can be
formulated using a wide range of core and coating materials. The technology is
versatile enabling the encapsulation of solid, liquid or gaseous cores and can
be used for various applications. The
particles can be further processed into other dose forms and compressed for
example in conventional tabletting environments.
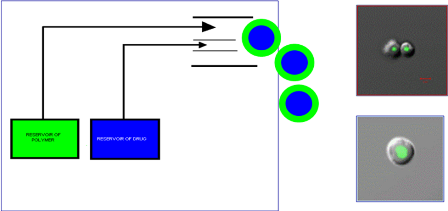
Figure
1. Schematic of RCSI’s novel MPE technology and electron micrographs of 1 micron
particles showing a fluorescent core in a biodegradable polymer
coat
Applications
There
are many opportunities to apply this novel MPE technology including enhancing
low solubility, bioavailability, short duration of action and poor
stability. This technology is
especially suited to insoluble compounds where small free-flowing drug particles
coated with a surface stabiliser are desirable to enhance drug re-dispersibility
and dissolution. In addition the
technology has a niche application in the sustained delivery of therapeutic
agents in particular of biological therapeutics (figure 2). RCSI’s MPE
technology has also been shown to enhance uptake across cells and the blood
brain barrier in in vitro cell models
and in vivo pre-clinical models and is suitable for targeted delivery of
biological therapeutics including
vaccines.
Figure
2. Cumulative release of Trastuzumab from microcapsules, < 5microns, prepared
using RCSI’s MPE technology

Advantages
- Versatile technology suitable for the development of
microencapsulated materials for application to pharmaceutical,
food, cosmetic and other industries
- New proprietary method for formulation of submicron surface
stabilized particles suitable for solubility enhancement of active
pharmaceuticals
- Suitable for formulation of low density/hollow micro and nanoparticles for pulmonary or diagnostic applications
- Suitable for formulation of microparticles with programmable elease
and targeted delivery
- Allows for enhanced systemic delivery of actives including
biological therapeutics and vaccines
across bio/physiological
barriers
- Ease of scale up to manufacturing scale
Contacts:
Dr. Gearóid Tuohy, RCSI Technology Transfer, 123 St Stephen’s Green, Dublin 2,
Ireland.
Email: gearoidtuohy@rcsi.ie Tel: +353 1
4022362
Principle Investigator:
Dr. Zeibun Ramtoola, School of Pharmacy, Royal College of Surgeons,
123 St Stephen’s Green, Dublin 2, Ireland. Email: zramtoola@rcsi.ie Tel:
+353 1 40228626 or +353 1 4022498