Description:
Introduction
Solid
dosage forms for oral drug administration are the mainstay of product
formulation. Conventionally a company will look at this strategy as a convenient
and reproducible method of manufacture. This does always however take into
account patient needs and preferences and undoubtedly a significant number of
patients find difficulty in swallowing a tablet. Convenience and palatability of
the dosage form are major considerations and orally disintegrating tablets have
addressed this in a number of cases. Currently available technologies for oral
disintegration are based upon effervescent systems, freeze-dried matrices or
rapidly-disintegrating granulated material. A major limitation of current
technologies is their low mechanical strength inherent to their fast disintegration in the
mouth. This results in the requirement of specialized costly packaging to
protect the tablets and maintain them intact for administration.
The oral drug delivery market is a $35bn industry and expected to
grow as much as 10% per year. There is a clear opportunity for new enhanced oral
products arising within this market segment. With a growing elderly population and associated chronic diseases
such as diabetes, cardiovascular diseases, Parkinson diseases, oral technologies
and products which offer ease of administration and cost benefit are expected to
stimulate the market for oral delivery.
Non-invasive delivery of insulin and GLP-1 analogs for type II
diabetic patients in particular an oral or buccal formulation represents a
significant unmet need for these patients. The global market for diabetes therapeutics was valued at $97bn in
2008, (with over 200m people affected), and is forecast to grow to $113bn by
2013
Technology
The
School of Pharmacy at RCSI has developed a novel technology which produces
mechanically strong tablets, with low friability and can withstand conventional
blister-packing. The manufacture utilises existing tablet presses, is cost
effective and as the manufacturing process avoids liquids, it is suitable where
stability may be a consideration. The technology allows incorporation of
microparticulates to produce extended-release and taste-masked, orally
disintegrating tablets. Importantly
RCSI has shown that the major excipient used in its ODT technology has an effect
on cell membrane permeability, improving the absorption of agents administered
in this way. RCSI is currently examining adding further enhancers to increase
the absorption-enhancing effect of the formulation. One important consequence of
this is the use of the technology in the delivery of certain large molecules,
including peptide agents that are otherwise destroyed after swallowing.
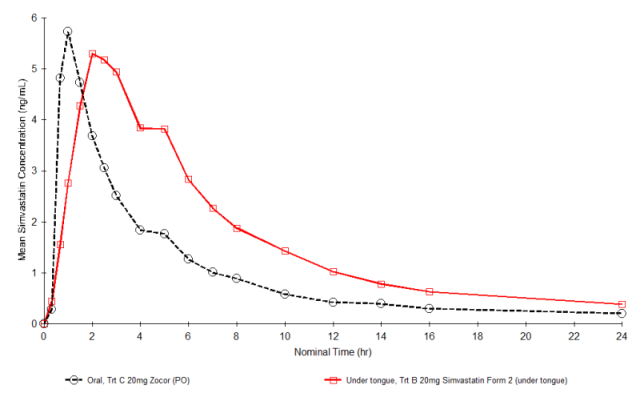
Evaluation in a phase I pharmacokinetic study in human volunteers
(n=18) of RCSI’s simvastatin formulation showed a 50% increase in
bioavailability when administered sublingually compared to oral administration
of the
innovator product, ZocorTM (
Figure 1).
Figure
1. Pharmacokinetic profiles of Simvastatin from RCSI’s simvastatin ODT formulation administered
sublingually vs Zocor® tablet administered orally.
Applications
There
are many opportunities to apply this approach to new actives or to extend life
or add value to existing actives. This technology is
especially suited to relatively potent agents for which rapid onset of action is
desired, for example in providing pain relief, relief from nausea, sleep or
sedation, or cardiovascular effects. In addition the technology
has a niche application in the buccal delivery of therapeutic agents.
Delivery through the buccal mucosa enters the systemic circulation directly
thereby avoiding first pass metabolism and offering an added advantage for
therapeutic agents with poor permeability and oral bioavailability.
Oral
mucosal delivery has been shown to be a potential route for insulin delivery,
with detectable insulin concentrations and increased glucose consumption
following delivery as an aerosol to the oropharyngeal cavity [1, 2]. While oral
sprays show indications of systemic delivery, this will be limited by mucosal
contact time. A buccal delivery system will produce enhanced contact time. RCSI
is currently seeking a partner to help bring this product to a clinical study.
The increasing prevalence of type 2
diabetes makes this an area of significant value.
Advantages
- New proprietary method allows for enhanced systemic absorption of
actives from buccal mucosa
- Versatile technology suitable for the development of enhanced
products for veterinary medicines, OTC, Rx medicines and line
extensions
- New proprietary method allows the incorporation of
microencapsulated drugs for enhanced bioavailability, flexibility of dosing
and immediate and/or controlled release for superior therapeutic
benefit.
- Lower production, packaging and distribution costs compared to
current commercially available products
References
- Cernea
S, Kidron M, Wohlgelernter J, Raz I. Dose-response relationship of an oral
insulin spray in six patients with type 1 diabetes: a single-center,
randomized, single-blind, 5-way crossover study. Clin Ther. 2005
Oct;27(10):1562-7
- Cernea
S, Kidron M, Wohlgelernter J, Raz I. Dose-response relationship of an oral
insulin spray in six patients with type 1 diabetes: a single-center,
randomized, single-blind, 5-way crossover study. Clin Ther. 2005
Oct;27(10):1562-70
Contacts:
Dr. Gearóid Tuohy, RCSI Technology Transfer, 123 St Stephen’s Green, Dublin 2,
Ireland.
Email: gearoidtuohy@rcsi.ie Tel: +353 1
4022362
A Method of Producing a Fast Dissolving Tablet
PCT Date: 03/04/2008
Orodispersible Tablets
PCT Date: 27/03/2010
Orodispersible Dosage Forms Containing Solid Drug
Dispersions
PCT Date: 18/05/2010
Principle Investigator:
Dr. Zeibun Ramtoola, School of Pharmacy, Royal College of Surgeons,
123 St Stephen’s Green, Dublin 2, Ireland. Email: zramtoola@rcsi.ie Tel:
+353 1 40228626 or +353 1 4022498