Description:
Introduction
Novel technology developed at RCSI has produced an improved, facile,
low temperature and “green” technology for the production of racemic sulfonic
acids. Sulfonic acids are precious compounds that are present in a wide number
of marketed compounds such as taurine (used as a functional food in many energy
products and drinks, e.g., Red Bull) and saclophen (used in anti-spasmodic drugs
such a Lioresal). At present, their
production involves the reaction of alkenes and bisulfite at high temperature
and in the presence of radical initiators. The involvement of radical species
raises the risk of explosions and increases the costs of manufacture. The
proposed chemistry runs at room temperature and makes no use of radical
initiators. Additionally, the catalysts used are simple, inexpensive amines that
could be recovered and recycled at the end of the reaction by acid base wash.
Consequently, the technology allows for the preparation of sulfonic acids
without the need for heating-cooling equipment and peroxides as radical
initiators, making the preparation of the title compounds safer, cleaner and
more cost effective.
Technology
Amines in general and triethylamine in particular have been found to
enhance the addition of bisulfite to alkenes. Novel reaction conditions
conducted at RCSI have allowed the addition of bisulfite to alkenes to occur at
room temperature and without the need for radical initiators. A protocol leading
to enantio enriched sulfonic acid has now been developed for the first
time.
This novel technology provides:
1.
A “green” and practical methodology to prepare sulfonic acids that
are active ingredients in surfactants and commercially available soaps.
2.
A novel methodology producing sulfonic acids that are not available
using conventional methodologies, useful for the preparation of drug like
compounds.
3.
A method to prepare sulfonic acids in enantioselective pure
form.
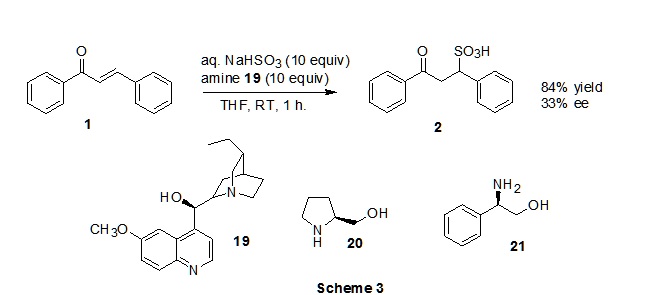
Example of enantioselective addition of bisulfite to activated
alkenes
Applications
The invention may be exploited as a process to manufacture existing
compounds for example, taurine or saclophen; or as a technique to prepare
end-products, for instance chiral sulfonic acids could be used in the
preparation of enantiopure amines.
At present, two steps are required for the production of enantiopure
sulfonic acids (addition of bisulfite to alkenes and subsequent resolution).
RCSI’s technology has the potential to supply customers with a method to obtain
enantiopure sulfonic acids directly from alkenes using mild conditions. The
families of enantiopure sulfonic acids prepared in this way will be applicable
to drug discovery and the preparation of organic intermediates and, as such,
represents a platform technology
for the use of sulfonic acids. For example, sulfonic acids are “interchangeable”
with carboxylates and tetrazoles, two templates often present in active
ingredients. Hence, the technology proposed could enlarge the scope of
investigation of many medicinal chemistry projects involving these structural
elements.
Advantages
Advantages of this technology include excellent solvency and
compatibility, rich and fine foam production, ease of biodegradation, low
toxicity and low irritation to skin. In the application of non-phosphorus
detergents, the technology provides for both washing ability and compatibility
with enzyme agents. Powder (grain) shape products have good fluidity, therefore
they are widely used in non-phosphorus washing powder, liquid detergents and
home washing products, textile, printing and dyeing industry, petrochemical
products, and industrial hard surface cleaning agents.
The families of enantiopure sulfonic acids prepared in this project
will be of interest for those involved in drug discovery or in the preparation
of organic intermediates and in pharmaceuticals R&D.
|
Feature |
Benefit |
|
No need for radicals |
Increased safety during manufacturing
processes |
|
Low temperature reaction |
Reduced cost of manufacturing |
|
Catalysts are reusable |
Cheaper and for environmentally friendlt
process |
Contacts:
Dr. Gearóid Tuohy, RCSI Technology Transfer, 123 St Stephen’s Green, Dublin 2, Ireland.
Email: gearoidtuohy@rcsi.ie Tel: +353 1 4022362
Dr Aoife
Gallagher, RCSI Technology Transfer, 123 St Stephen’s Green, Dublin 2, Ireland.
Email: aoifegallagher1@rcsi.ie. Tel: +353 1 4022394
Dr Liz Moran,
Enterprise Ireland,
East Point Business Park, Dublin 3.
Email: liz.moran@enterprise-ireland.com.
Tel: +353 1 7272696
Principle Investigator:
Dr. Mauro Adamo,
Department of Pharmaceutical & Medicinal Chemistry, Royal College of
Surgeons, 123 St Stephen’s Green, Dublin 2,
Ireland. Email: madamo@rcsi.ie. Tel:
+353 1 4022208