UDCA as a Treatment for Diarrhoeal disease
Description:
Introduction
The global impact of diarrheal disease: Diarrhoeal diseases represent a huge global burden. In
developing countries infectious diarrhoea kills 2.5 million children annually
while in Western societies diarrhoea is a feature of many intestinal disorders
including infectious diseases (ID), inflammatory bowel diseases (IBD), irritable
bowel syndrome (IBS), celiac disease, and a number of conditions associated with
bile acid malabsorption. It has been estimated by the American
Gastroenterological Association that ID, IBD and IBS alone represent an annual
cost to the US economy of more than $9.6 billion (€17 billion in Europe) in
terms of healthcare and lost work hours. Diarrhoea is also a prominent and
dose-limiting side effect of many chemotherapeutic agents, thereby limiting
their therapeutic efficacy. However, despite the prevalence and impact of
diarrheal diseases on global health there is still a lack of specific and
effective therapeutics and no drugs to treat diarrhea directly at the level of
dysregulated fluid transport currently exist.
Intestinal fluid movement occurs across the epithelial
cells and is driven by ion transport processes that establish osmotic gradients.
In the intestine, Cl- ion secretion is the primary driving force for fluid
secretion and direct modulation of the molecular components of the epithelial
secretory pathway therefore represents a logical approach for the development of
new therapeutics for diarrheal disease. However, drugs that act specifically in
this fashion are not yet available.
Technology
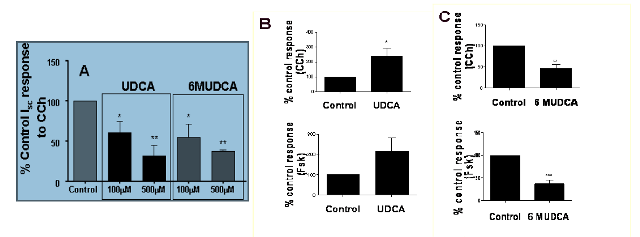
Analysis of the
effects of UDCA and 6-MUDCA on Cl- secretion in vitro and in vivo.
Cl- secretory
responses to prototypical secretagogues, carbachol (CCh) and forskolin (FSK),
were measured as changes in Isc across voltage-clamped T84 colonic epithelial cells or ex vivo
mouse colon. A) UDCA and 6-MUDCA were equipotent in
inhibiting Cl- secretory responses to CCh
in T84 cells while B) intraperitoneal
injection of UDCA (100 mg/kg) to mice enhanced responses to both CCh and FSK.
C) In contrast, treatment of mice with 6-MUDCA potently
inhibited agonist-induced colonic secretory responses.
Metabolically stable analogues of ursodeoxycholic acid
for treatment of diarrheal diseases. Ursodeoxycholic acid (UDCA) has been
used in Traditional Chinese Medicine for 1000’s of years in treatment of a
variety of ailments. More recently UDCA has found a place in Western medicine
for treatment of liver disorders and it is currently under investigation for a
possible therapeutic role in several other conditions. RCSI studies have
revealed a novel role for UDCA in inhibiting colonic epithelial Cl- secretion,
suggesting it might also be useful in treating diarrhea. However,
counter-intuitive to its antisecretory actions in vitro, when it is
used in clinical practice UDCA has the tendency to cause diarrhea. Our data
suggest that this is likely due to the fact that in vivo UDCA is
rapidly metabolised in the colon to lithocholic acid (LCA) which, in contrast to
UDCA, we have found to enhance epithelial secretory capacity. This invention
provides a new method to treat diarrhoeal diseases through the use of
metabolically stable analogues of UDCA that cannot be converted to LCA in the
colon.
Applications
Metabolically stable analogues of UDCA, typified by 6-methyl-UDCA
(6-MUDCA), exert potent antisecretory effects on colonic epithelial cells
in
vitro and in
vivo. Further RCSI studies have identified these
antisecretory actions as being mediated at the molecular level through
inhibition of specific transport proteins that comprise the epithelial
Cl- secretory pathway. Thus, stable
derivatives of UDCA may represent a new class of antidiarrheal drug that acts
directly at the level of the epithelial Cl- secretory mechanism.
Advantages
UDCA is already used extensively to treat liver disease and is known
to be very safe with few side effects. Stable analogues of UDCA have already
been synthesised elsewhere and, on the basis of our data, these compounds may
provide the first class of anti-diarrheal drugs that act directly at the level
of epithelial secretory processes.
|
Feature |
Benefit |
|
Directly targets intestinal epithelial secretion |
Avoids unnecessary side effects of indirect treatments eg
constipation, bloating, central effects,
etc |
|
Potentially useful in treating a wide range of diarrhoeal diseases of
different causes |
Large potential market |
|
Several stable analogues of UDCA already exist |
Facilitates development |
|
Unlike vaccines and oral rehydration solutions, drugs are easily
portable and do not require refrigeration. |
These are essential factors when considering distribution of a new
therapeutic for diarrhoeal disease in developing countries.
|
Contacts:
Dr Aoife Gallagher, RCSI Technology Transfer, 123 St Stephen’s
Green, Dublin 2, Ireland.
Email: aoifegallagher1@rcsi.ie. Tel: +353 1
4022394
Dr. Gearóid Tuohy, RCSI Technology Transfer, 123 St Stephen’s
Green, Dublin 2, Ireland.
Email: gearoidtuohy@rcsi.ie Tel: +353 1 4022362
Principal Investigator: Dr. Stephen Keely, Royal College of
Surgeons, ERC Beaumont, Dublin 2, Ireland.
Email:
skeely@rcsi.ie Tel: +353 1 8093821
Patent Information:
| Title |
App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
Patent Status |
|
|
|
Inventors:
Keywords:
|