Description:
Introduction
Every year approximately 1.2 million worldwide, are diagnosed with
breast cancer (International
Agency for Research). Currently women diagnosed with breast cancer
generally undergo
surgery to remove the tumour. Many will also receive either chemo
or radiotherapy or a
combination of both. Approximately two thirds of all breast cancers
are estrogen receptor
positive and it is these patients who will then receive endocrine
treatment for a further 5 years.
Though most patients initially respond to treatment, approximately
40% eventually relapse.
Why these patients develop resistance to the drug treatment is
unknown. Currently there is no
method of predicting which patients are likely to recur following
initial surgery. Clinical followup
of these women involves routine 6 monthly or annual checkups where
they are assessed.
Breast cancer recurrence is an insidious affliction as unlike the
primary tumour there is often
very little outward sign that all is not well until the disease has
progressed.
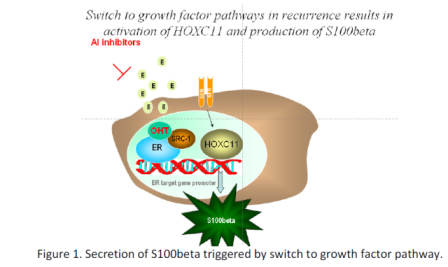
The Technology
Translational studies at RCSI have shown that the SRC1 associated
proteins HOXC11 and
S100beta are strong predictors of poor disease-free survival in
breast cancer patients (n=560).
This translational study identifies a biomolecular interaction
network central to the adaptive
response to endocrine therapy with clear clinical applications
(Fig.1). Disease recurrence
precipitates cessation of the regime and the initiation of second
line therapy, often with tyrosine
kinase inhibitors (TKIs). Early detection of breast disease
recurrence with S100beta prior to
clinical manifestation may detect the switch from endocrine to
growth factor dependent breast
cancer hence these biomarkers have the potential to provide
clinical information regarding the
timely commencement of TKI therapy.
The proposed immunohistochemical test detects the presence of
HOXC11 or S100beta in tissues taken at the time of initial surgery, this represents a
statistically significantly predictor of disease-free survival independent of treatment (p<0.0001; hazard ratio:
5.79). This association is significantly stronger than any of the currently used clinical
makers, including HER2. Monitoring of S100-beta, in patient’s blood could enable evaluation of their
response to current therapy and also facilitate improved drug selection if recurrence does occur. Levels
of S100beta will be taken preoperatively, postoperatively and every six months during the
survival period. S100-beta is a strong predictor of poor disease-free survival on endocrine
treatment (hazard ratio: 5.82; p<0.0001) and may be used as a serum marker of tumour progression (hazard
ratio: 5.3; p<0.001) (Fig.2) (McIlroy
et al Cancer Res 2010; 70;1585–94).

Applications
These biomarkers have the potential to provide clinical information
regarding the timely commencement of TKI therapy, providing an opportunity for a
partnership between RCSI and a pharmaceutical company or diagnostic test manufacturer to develop a
companion diagnostic for the timely use of these drugs for breast cancer.
Advantages
·
Accurate
assessment of a tumour at the time of initial surgery
-Informs clinicians of patients likely to
relapse
-Identifies
endocrine patients who may benefit from combined TKI treatment in the absence of
initial growth factor
receptor status
·
Ongoing
assessment of disease free survival status in breast cancer patients
undergoing
treatment
permitting a timely alteration of drug if required.
·
Detection
of recurrence or metastasis in tumours that no longer respond to front line
treatment.
·
Very
strong hazard ratio; better that Her2, Mammaprint and Oncotype
Dx
·
Test
can be easily performed in hospital labs without the requirement to ship
samples.
Contact:
Dr
Aoife Gallagher, RCSI Technology Transfer, 123 St Stephen’s Green, Dublin 2,
Ireland.
Email:
aoifegallagher1@rcsi.ie. Tel: +353 1 4022394